In recent years, several major computer manufacturers have made headlines by recalling lithium ion (Li-ion) laptop batteries because of an unfortunate tendency of some of these batteries to burst spontaneously into flames. While rare, spontaneous blazes caused by Li-ion batteries can obviously be quite dangerous. As these batteries proliferate—today they power millions upon millions of devices ranging from smart phones and tablets to small appliances and even hybrid automobiles—the motivation is strong to develop foolproof methods of fire prevention. Now researchers at a DOE Energy Frontier Research Center (EFRC) have proposed a potential new approach to preventing such fires, drawing on one of the more interesting trends in contemporary materials science—the development of “self-healing” materials.
 Enlarge Photo
Enlarge Photo
 Photo courtesy of Scott R. White
Photo courtesy of Scott R. White
Microscopic image of heat-sensitive microspheres designed to melt at elevated temperatures, shutting down a lithium ion battery in the event of overheating.
For the most part, of course, today’s laptops are quite safe, and the recalls represent only a tiny fraction of total laptops sold. But while manufacturers have made great strides in improving batteries to ensure against fires, it could be argued that the fire hazard remains almost inherent in a Li-ion battery’s fundamental design. At its most basic level, each Li-ion cell has three thin layers—a positive electrode made of a lithium alloy, a negative electrode made of carbon, and a third thin plastic or polymer layer between the two known as a “separator.” The separator performs two basic functions. First, of course, it keeps the positive and negative electrodes apart, preventing a short circuit. Second, it has tiny pores that permit lithium ions to flow between the two electrodes (from the positive to the negative electrode when charging, and in the reverse direction when discharging). The separator is bathed in a (typically flammable) liquid electrolyte such as ether, through which the lithium ions flow.
A problem arises if the separator becomes damaged or destroyed, causing the electrodes to short-circuit and overheat, initiating a chemical reaction between the electrolyte and the electrode. Such a short circuit can trigger a “thermal runaway” whereby the increase in temperature of the cell accelerates the chemical reaction, adding to the heat in turn and leading to the combustion of battery materials. Ultimately such a failure can trigger a chain reaction whereby successive cells in the battery burst into ever hotter flames. (If you poke around the web, it’s easy to find some rather dramatic footage of laptop battery fires—usually with the warning, “Don’t try this at home!”) Such failures can also be triggered by mechanical injury, by overcharging, or by overheating from an external source.
That’s why today’s separators are generally designed to perform an additional safety function. Typically composed of a bilayer structure of polyethylene (PE) and polypropylene (PP) or a trilayer PP-PE-PP arrangement, the separators are designed to soften at a critical high temperature, closing the pores through which the lithium ions travel and thereby shutting down battery operation. The problem is that under these hot conditions, the separator also tends to shrink, raising the risk of contact between the electrodes. Moreover, if for some reason the temperature continues to climb, the separator may be entirely destroyed.
As an alternative approach, a team led by Scott R. White of the University of Illinois at Urbana-Champaign (UIUC) has drawn on nearly two decades of work in developing “self-healing” materials. White is a principal investigator in the Center for Electrical Energy Storage (CEES), an EFRC led by the U.S. Department of Energy’s Argonne National Laboratory, in which UIUC is a major partner. The research was reported in the journal Advanced Energy Materials, which also featured the work on its cover.
“
The ambition of the researchers has been to create materials that healautonomously in a manner mimicking organisms, as in the self-mending of a broken bone.”
While early research on self-healing materials dates back to the 1990s, the technique first began to make science headlines in 2001 with an article in the journal Nature co-authored by White and White’s longtime UIUC colleagues Nancy R. Sottos and Jeffrey S. Moore (both also principal investigators in CEES), along with several other researchers. The article described a method for automatic healing or repairing of microscopic cracks in materials made of polymer (typically plastics or plastic-like substances). As a result of normal wear and tear, materials made of polymers tend to develop internal microscopic cracks that can lead to failure—for example, loss of conductivity in electrical circuits or physical breakdown (say, as in the large crack that suddenly forms in your five-year-old tennis racket after a particularly hard shot).
White and his co-authors described an approach whereby they built hollow microscopic capsules into the material. Inside each capsule was a liquid “healing agent.” Embedded in the material outside the capsules, meanwhile, was a catalyst designed to react with the liquid. As cracks began to develop in the material, they would eventually encounter and burst a microcapsule. The liquid healing agent would be released from the burst microcapsule into the material and react with the catalyst to seal the crack. The authors described their approach as “biomimetic” and “inspired by biological systems.” Their ambition has been to create materials that heal autonomously in a manner mimicking organisms, as in the self-mending of a broken bone. The authors coined a name for this property: “autonomic healing.”
Creating self-healing materials, however, turns out to be a rather painstaking process. The stiffness of microcapsule’s surface needs to be exactly right—too stiff, and the cracks will travel around the microcapsule; insufficiently stiff, and the microcapsules will actually encourage cracking. It is probably too difficult a process for many applications. But in certain cases—for example, prosthetic parts implanted by surgery in the human body that need to endure for many years—self-healing properties that substantially extend the lifetime of a material could have major benefits.
 Enlarge Photo
Enlarge Photo
 Photo courtesy of Scott R. White
Photo courtesy of Scott R. White
Schematic of concept for lithium ion battery shutdown through melting of microspheres. Melted microspheres shut down battery operation by blocking pores through which lithium ions pass.
In the recent work on batteries, White, Sottos, Moore, and colleagues applied a similar technique to preventing Li-ion thermal runaway. The technique was to coat either the anode layer or separator layer with heat-sensitive “microspheres,” or tiny solid capsules, ranging from two to forty-some nanometers in diameter. When the battery reached a certain temperature, the microspheres would melt, blocking the pores in the separator through which lithium ions pass and shutting down battery operation. The researchers fabricated two sets of microspheres, one made of polyethylene and another of paraffin wax. They tested the system using coin-shaped CR2032 Li-ion batteries (the type you find in the keyless remote to your car). They experimented with different levels of coating to maximize both battery operation at room temperature and the effectiveness of shutdown at high temperatures. They were able to demonstrate shutdown (reduced charge capacity by 98 percent) at 110º Celsius with PE microspheres and shutdown at 65º C with the paraffin wax, while sustaining normal battery operation at room temperature. The safety advantage is that these triggering temperatures are considerably lower than the melting point of the two materials in the separator (PE at 130º C. and PP at 165º C.), so battery operation is shut down with the separator still safely intact. Examination of the surfaces with scanning electron microscopy confirmed the melting pattern.
The one key weakness of the experimental system is that shutdown took longer to unfold than it would normally take for a Li-ion battery to go critical. For example, shutdown with the PE microspheres took six seconds, while a battery could go critical in just one second. But having demonstrated the concept, researchers are prepared to look at alternative polymer materials, with more rapid responses, as well as alternative “triggers,” since, as the researchers noted, “Microspheres can be engineered to respond to a variety of stimuli, including pressure, pH, electric fields, magnetic fields, and temperature.”
So the hope is that the choice of material can be fine-tuned eventually to achieve the desired performance and to expand the application of the system to other types of batteries. “Since the melt transition temperature . . . dictates the triggering temperature of the cell shutdown,” the researchers wrote, “this highly customizable mechanism should be applicable to a wide variety of battery chemistries and their unique shutdown requirements.” The work was supported primarily by the DOE Office of Science.
—Patrick Glynn, DOE Office of Science, Patrick.Glynn@science.doe.gov
Funding
DOE Office of Science, Office of Basic Energy Sciences
American Association of University Women
University of Illinois at Urbana-Champaign College of Engineering
Publications
Marta Baginska, Benjamin J. Blaiszik, Ryan J. Merriman, Nancy R. Sottos, Jeffrey S. Moore, and Scott R. White, “Autonomic Shutdown of Lithium-Ion Batteries Using Thermoresponsive Microspheres,” Advanced Energy Materials 2, 583 (2012).
Lynn Trahey, “Better Safe than Sorry: Highly customizable polymer microspheres effectively prevent battery operation at dangerous temperatures,” Frontiers in Energy Research: Energy Frontier Research Center Newsletter, October 2012; available at http://www.energyfrontier.us/newsletter/201210/better-safe-sorry .
.
S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown, and S. Viswanathan, “Autonomic healing of polymer composites,” Nature 409, 794 (2001).
Magdalena Helmer, “Plastic, heal thyself: fatigued materials have a self-help cure,” Nature, published online on February 15, 2001.
Related Links
Center for Electrical Energy Storage
Autonomous Materials Systems, University of Illinois at Urbana-Champaign
DOE Energy Frontier Research Centers
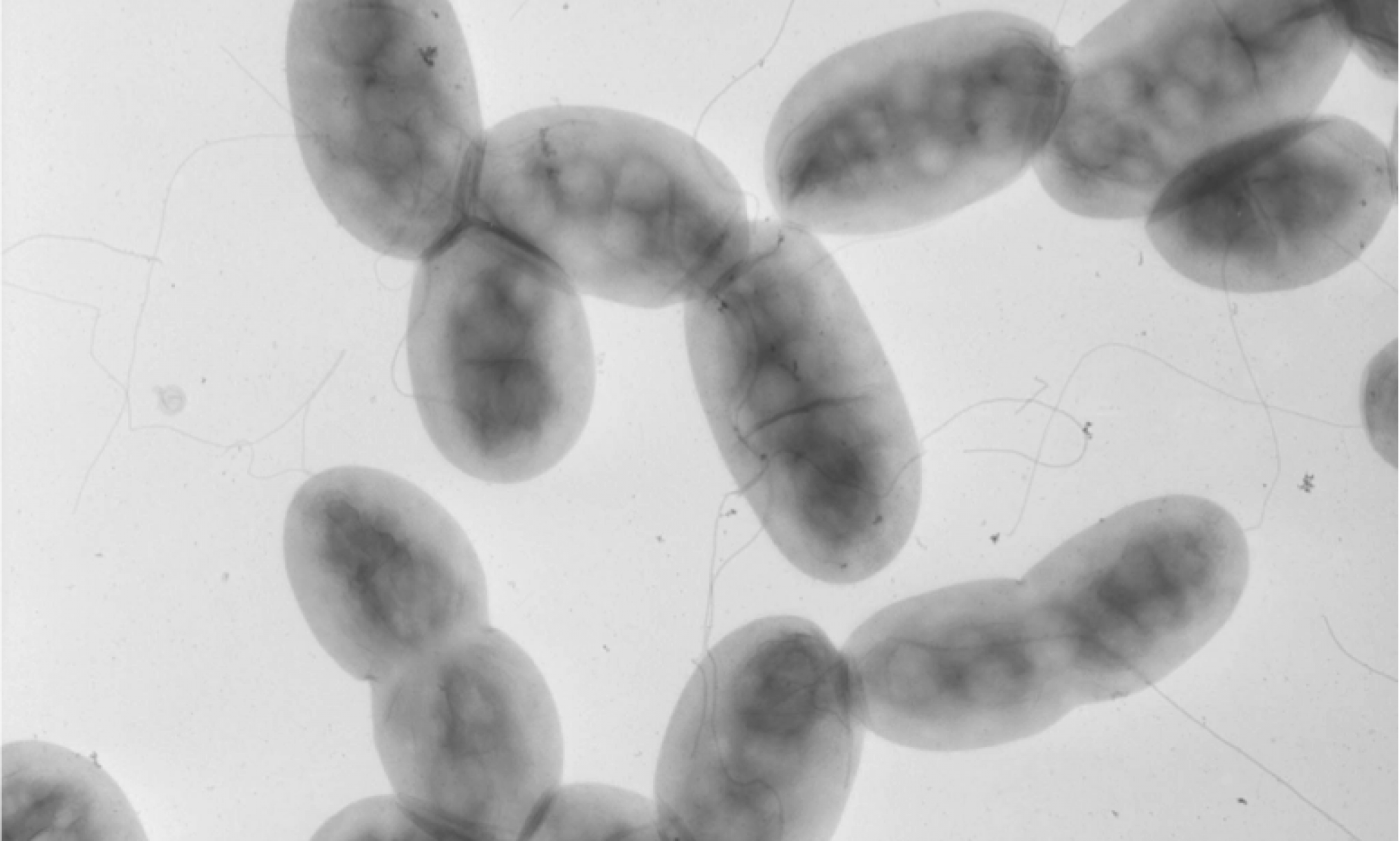

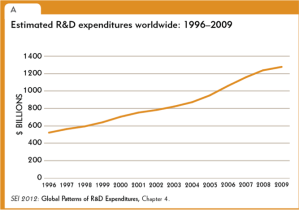
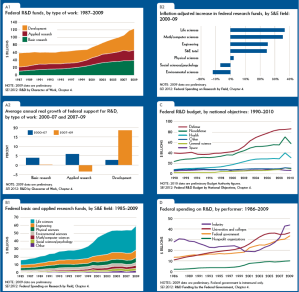
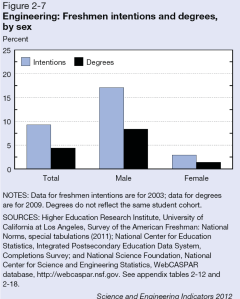
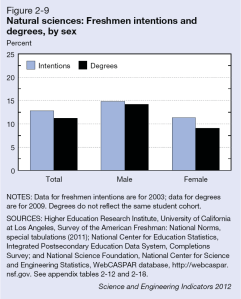
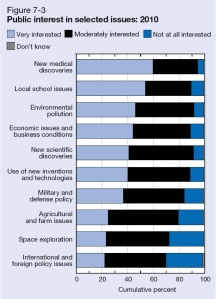
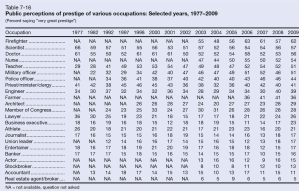
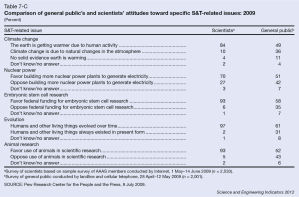
 Photo courtesy of Scott R. White
Photo courtesy of Scott R. White Photo courtesy of Scott R. White
Photo courtesy of Scott R. White